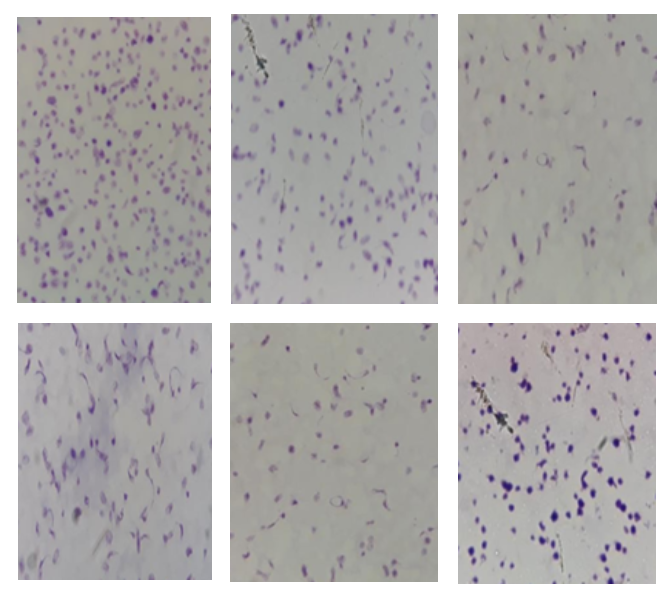
Platelet Morphological Assessment Based on Shelf-Life of Concentrated Platelet Components
Abstract
Concentrated platelet components are the components or platelets that are processed through centrifugation from whole blood or a single donor. The components of the concentrate platelets are stored in an agitator at a temperature of 20-24◦C with a maximum shelf-life of five days. During the storage, a change in component metabolism occurs, which is characterized by alteration in platelet morphology as an indicator of decreasing quality of platelet components. The purpose of this study was to determine the quality of the components of platelets based on the shelf-life by measuring the platelet morphological values with the Kunicki method. This study applied an experimental method by conducting inspections of the regional blood transfusion unit (UTDD) of the Indonesian Red Cross of Jakarta Capital Special Region (PMI DKI Jakarta). Platelet morphological values of six components of platelets were examined by the Kunicki method on day 0 to day 5 of shelf-life. Quality assessment was carried out based on a significant difference-dependent t-test and comparison with normal value. The results of the study by performing statistical analysis of the significant difference-dependent t-test showed a significant difference of values between day 0 and day 1 to day 5 of shelf-life. Comparison with normal values depicted that all samples stored on day 4 had a morphological value of more than 200. On day 5 of shelf-life, the platelet morphology values of five samples were below 200. The quality of the concentrated platelet components is said “good” if it is above 200. This study concluded that there was a significant difference in the platelet morphology values between the components of concentrated platelets on day 0 and day 1 to day 5 of shelf-life. All samples were of good quality until day 4 of storage. However, on day 5 of storage, only one sample was good in quality.
References
Bethesda, M. (2005) Association American Blood Bank Technical Manual 15th Edition. AABB. USA. USA.
Dewland, N. (2017) Does an Objective Laboratory Measurement of Platelet Quality Correlate with Clinical Efficacy?Departement of Biological and Medical Sciences. Oxford Brookes University. Headington. Oxford Brookes University. Headington.
Diyanti, Luh Putu Sukma, S. H. and I. W. P. S. Y. (2017) ‘Perbedaan Kadar Glukosa Konsentrat Trombosit Pada Penyimpanan Hari I,III,V di Unit Donor Darah PMI Provinsi Bali/RSUP Sanglah Denpasar.’, E-Jurnal Medika, Edisi Mare, pp. 1-5.
Harmening, M. D. (2012) Modern Blood Banking & Transfusion Practices. 6th edn. Philadelphia: F.A. Davis Company.
INFODATIN (2014) Situasi Pelayanan Darah di Indonesia. Jakarta.
INFODATIN (2018) Pelayanan Darah di Indonesia. Indonesia.
Jain A, Neelam Marwaha, Ratti Ram Sharma, Jyotdeep Kaur, M. T. and H. K. D. (2015) ‘Serial Change in Morphology and Biochemical Markers in Platelet Preparations with Storage’, Asian Journal of Transfusion Science, Januari-, pp. 41 – 47.
Kunicki, TJ, M. Tucelli, G.A Becker, and R. . A. (1975) ‘A Study of Variable Affecting the Quality of Platelets Stored at “Room Temperature”.’, Transfusion, Sept-Oct, pp. 414–421.
Levin,E, Craig Jenkins, Brankica Culibrk, Maria IC, Gyongyossy-Issa, Katherine Serrano, and D. V. D. (no date) ‘Development of a quality monitoring program for platelet components: a repost of the first four years experience at Canadian Blood Services.’, UBC Centre for Blood Research., Edisi Mei, pp. 1 – 9.
Mittal, K. and Kaur, R. (2015) ‘Platelet storage lesion: An update’, Asian Journal of Transfusion Science, 9(1), pp. 1–3. doi: 10.4103/0973-6247.150933.
Raveendran, R. et al. (2019) ‘The Effect of Storage on Platelets in Platelet Rich Plasma and Platelet Concentrate’, International Journal of Contemporary Medical Research [IJCMR], 6(1). doi: 10.21276/ijcmr.2019.6.1.34.
Ravindra, P. S. ; N. M. ; P. M. ; S. D. (2009) ‘Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods’, Asian Journal of Transfusion Science, 3 (2). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2920479/?report=reader.
Rukman, K. (2014) Hematologi dan Transfusi. Erlanggamedical series.
Seong-Hoon Yun, Eun-Hye Sim, Ri-Young Goh, Joo-In Park, J.-Y. H. (2016) ‘Platelet Activation: The Mechanisms and Potential Biomarkers’, BioMed research international. Available at: http://downloads.hindawi.com/journals/bmri/2016/9060143.pdf.
Six, K. R., Compernolle, V. and Feys, H. B. (2020) ‘Platelet biochemistry and morphology after cryopreservation’, International Journal of Molecular Sciences, 21(3). doi: 10.3390/ijms21030935.
Snowball, M. A. O. (2016) Comparison of Platelet Storage Lesions between Leukoreduced and Non-Leukoreduced Canine Platelet Concentrate. University of Guelph. Canada.
Tynngård,N., Wallatesdt,M., Södergren,L.A., Faxälv,L., and Ramström, S. (2014) ‘Platelet adhesion changes during storage studied with a novel method using flowcyometry and protein-coated beads’, Informa Healtcare, pp. 3–4.