Subacute Toxicity Test of (Scaevola taccada Gaertn.) Roxb Leaf Extract on Kidney and Liver Function in Rats (Rattus norvegicus)
Abstract
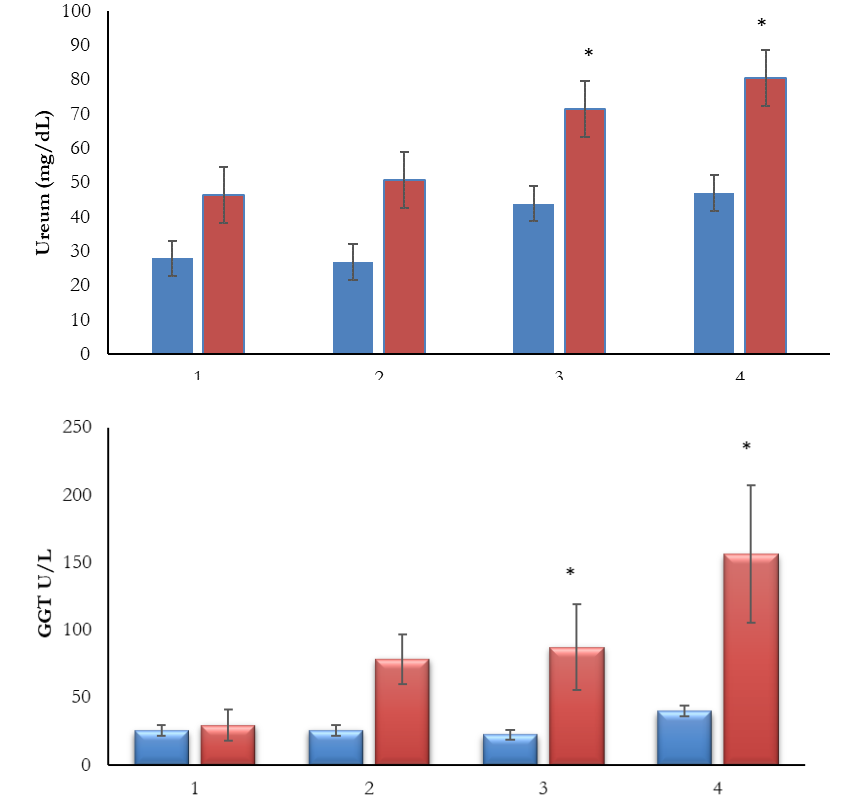
Beruwas laut leaf (Scaevola taccada (Gaertn.) Roxb has pharmacological effects such as anti-inflammatory, analgesic, antioxidant, antidiabetic and anticancer. Although beruwas laut leaf have many advantages, safety is the main requirement that herbal medicine must have. This research was aimed to prove sebacute of beruwas laut leaf (Scaevola taccada (Gaertn.) Roxb.) about the function of kidney and liver. This research used 20 rats were devided into 4 groups. Group 1 as a control group and groups 2,3, and 4 as an experimental group by administration of beruwas laut leaf extract with dose 200 mg/kgBB, 400 mg/kgBB, and 600 mg/kgBB. The extract was made using maceration and sebacute toxicity testing was carried out for 14 days. After giving the extract, some of rats had diarrhea. The results showed significant effect to increase levels of ureum and CGT after administration of dose 400mg/kgBB. Moreover, administration of dose 600 mg/kgBB caused significant effect in liver biomarkers and kidney (GGT and ureum). It was concluded that ethanol extract of beruwas laut leaf (Scaevola taccada (Gaertn.) Roxb.) with dose 200mg/kgBB showed safe but toxic to kidney and liver with dose 600mg/kgBB.
References
Depkes RI. 2000. Parameter Standar Umum Ekstrak Tumbuhan Obat. Jakarta: Departemen Kesehatan Republik Indonesia. hal. 1,5,10-11
Hendriani, R, 2007, Uji toksisitas subkronis kombinasi ekstrak etanol buah mengkudu (Morinda citrifolia Linn.) dan rimpang jahe gajah (Zingeber offcinale Rosc.) pada tikus wistar, Jatinangor, Fakultas Farmasi, Universitas Padjajaran.
Lu, Fank. C. 1995. Basic Toxicology. Fundamentals, Target Organs, and Risk assessment. Hemisphere Publishing. New York, NY (USA).
Makris K, Spanou L. 2016. Acute Kidney Injury : Definition, Pathophysiology and Clinical Phenotypes.
Murtie, Afien. 2013. Kupas Tuntas Pengobatan Tradisional. Trans Idea Publishing: Jogjakarta.
Rahmawati. 2013. Aktivitas Antioksidan Ekstrak Daun beruwas laut (Scaevola taccada (gaertn.)Roxb) Pada Tikus Putih Diabetes. jurnal JST Kesehatan Bagian kedokteran Universitas Hasanuddin, Makassar. Vol.3 No.4 : 313–319.
Widyati, 2014, Praktik Farmasi Klinik Fokus pada Pharmaceutical Care, Surabaya